In chemistry, a valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond. In a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. For instance, hydrogen atoms have one valence electron, while oxygen atoms have two. That is why a water molecule contains one oxygen and two hydrogen atoms that are connected by singles covalent bonds:
|
But let us consider a more complex example: bifluoride. Bifluoride is an inorganic anion with the chemical formula [HF2]−.
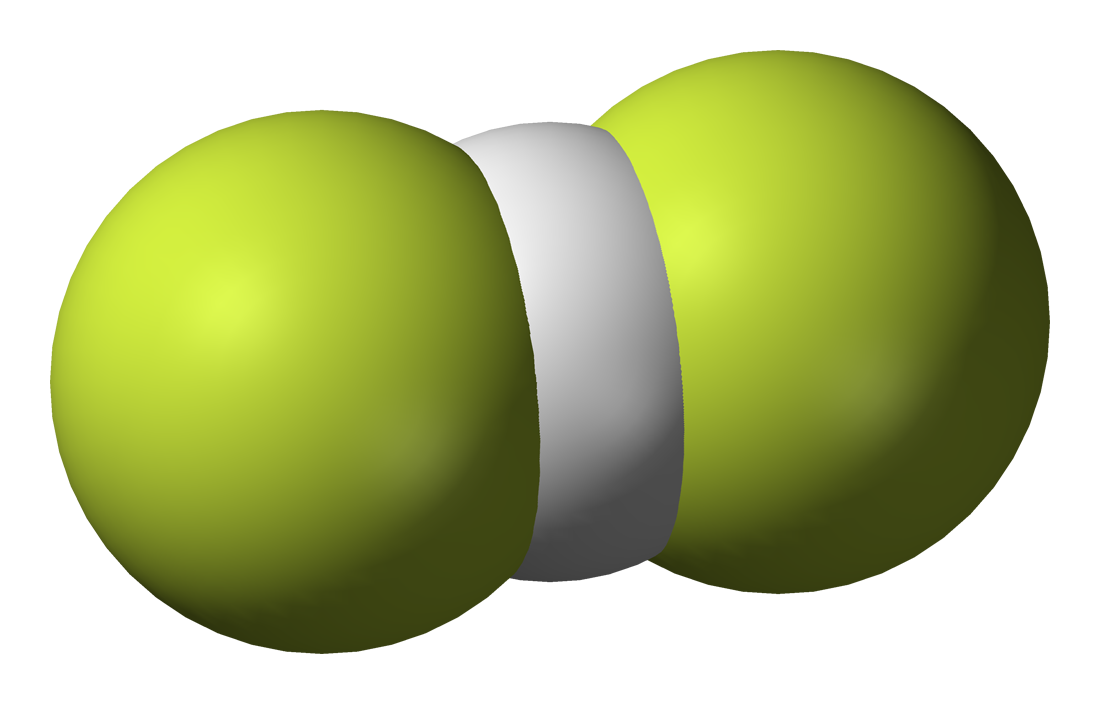 | |
| Public Domain, https://commons.wikimedia.org/w/index.php?curid=1496287 |
It is not a strange anion. Some [HF2]- salts are common, examples include potassium bifluoride (KHF2) and ammonium bifluoride ([NH4][HF2]).
As shown in the figure above, the structure of the anion is symmetric, with the hydrogen situated in the mid-point of the F-F distance. In addition, the H-F contacts in this ion are very short, like in covalent bonds, and the corresponding H.F interactions are also strong enough to be classified as covalent bonds. So the corresponding Lewis structure of the anion should be [F-H-F]-. But, how is this possible? Hydrogen atoms have one valence electron. They cannot be connected by two covalent bond!
Are you able to give an explanation? Accept the challenge!
Please, explain your reasoning. You can post your attempted answers in the comment box below. Please, do not use Facebook or Twitter to give your answers.
This comment has been removed by a blog administrator.
ReplyDelete